Differences Between a Continuous Spectrum an Emission Spectrum and an Absorption Spectrum
Key Difference: Emission is the ability of a substance to give off light, when it interacts with heat. Absorption is the opposite of emission, where energy, light or radiation is absorbed by the electrons of a particular matter.
Emission and absorption spectra are techniques that are used in chemistry and physics. Spectroscopy is the interaction of radiation and matter. Using spectroscopy, a scientist can figure out the composition of a certain matter. This is really beneficial, of dealing with unknown substances. Emission spectra and absorption spectra are different from each other but still related.
 Emission is the ability of a substance to give off light, when it interacts with heat. Every substances reacts differently when it interacts with light. The material starts off with being in the ground state, where all molecules are stable and settled. However when heat, energy or light is applied to a substance, some of the molecules transition into a higher energy state or an excited state. During this state the molecules are unstable and try to emit the energy in order to reach the state of equilibrium. The molecules emit energy in the form of photons or light. The difference between the substance in ground state and excited state is then used to determine the emission level of the substance.
Emission is the ability of a substance to give off light, when it interacts with heat. Every substances reacts differently when it interacts with light. The material starts off with being in the ground state, where all molecules are stable and settled. However when heat, energy or light is applied to a substance, some of the molecules transition into a higher energy state or an excited state. During this state the molecules are unstable and try to emit the energy in order to reach the state of equilibrium. The molecules emit energy in the form of photons or light. The difference between the substance in ground state and excited state is then used to determine the emission level of the substance.
Each element or substances has a unique emission level or the amount of energy it radiates; this helps the scientists identify elements in unknown substances. The emission of an element is recorded on an emission spectrum or atomic spectrum. The emittance of an object measures how much light is emitted by it. The amount of emission of an object varies depending on the spectroscopic composition of the object and temperature. The frequencies on a emission spectrum are recorded in light frequencies, where the color of the light determines the frequency. The frequencies can be determined using the formula Ephoton = hv, where 'Ephoton' is the energy of the photon, 'v' is its frequency, and 'h' is Planck's constant. Emission can happen in the form of light and rays, such as gamma and radio. The spectrum is a dark wavelength with bands of color on it, which is used to determine the emission of the object.
Absorption is the opposite of emission, where energy, light or radiation is absorbed by the 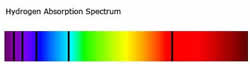 electrons of a particular matter. Absorption is the ability of a matter or electron to absorb light or radiation which makes them transition into a higher energy state. Absorption is used to determine the absorption level of certain objects and their ability to retain heat. Absorption spectrum is the plotting of the energy that is absorbed by an element or substance. Absorption can be plotted in a wavelength, frequency or wave number. There are two types of absorption: atomic absorption spectra and molecular absorption spectra.
electrons of a particular matter. Absorption is the ability of a matter or electron to absorb light or radiation which makes them transition into a higher energy state. Absorption is used to determine the absorption level of certain objects and their ability to retain heat. Absorption spectrum is the plotting of the energy that is absorbed by an element or substance. Absorption can be plotted in a wavelength, frequency or wave number. There are two types of absorption: atomic absorption spectra and molecular absorption spectra.
Absorption is used to determine the presence of a particular substance in a sample, or the quantity of the present substance in the sample. They are also used in molecular and atomic physics, astronomical spectroscopy and remote sensing. Absorption is primarily determined by the atomic and molecular composition of the material. They can also depend on temperature, electromagnetic field, interaction between the molecules of the sample, crystal structure in solids and temperature. In order to determine the absorption level of a substance, a beam of radiation is directed at the sample and the absence of light that is reflected through the object can be used to calculate the absorption. The absorption spectrum is usually light colored, with dark bands that run through it. These dark bands are used to determine the absorption of the object.
| Emission | Absorption Spectra | |
| Description | Emission is the ability of a substance to give off light, when it interacts with heat. | Absorption is the opposite of emission, where energy, light or radiation is absorbed by the electrons of a particular matter. |
| Subjects | Chemistry and Physics | |
| Purpose | Can be used as part of spectroscopy to figure out the composition of a certain matter. | Can be used as part of spectroscopy to figure out the absorption level of certain objects and their ability to retain heat. Can also used in molecular and atomic physics, astronomical spectroscopy and remote sensing. |
| Types | - | Atomic absorption spectra and molecular absorption spectra. |
| Effect on molecules | When a substance interacts with light, then some of its molecules absorb the heat from the light and get excited. This causes them to become unstable and they try to emit the excess energy to get back to normal. The excited molecules release the excess energy in the form of photons, also known at light particles. | When a substance interacts with light, then some of its molecules absorb the light or the radiation. The types of light wavelength that get absorbed can be mapped. |
| Result | The type of photons emitted helps figure out the type of elements that the substance is formed of, as each element or substances has a unique emission level or the amount of energy it radiates | The type of light wavelengths that are absorbed helps figure out how much quantity of a substance is present in the sample. |
| In simple terms | Emission spectra records wavelengths emitted by materials, which had been stimulated by energy before. | Absorption spectra records the wavelengths absorbed by the material |
| Looks like | Dark colored, with light bands that run through it. These light bands are used to determine the types of photons emitted by the object. | Light colored, with dark bands that run through it. These dark bands are used to determine the absorption of the object. |
| Units | The frequencies of emission can be determined using the formula Ephoton = hv, where 'Ephoton' is the energy of the photon, 'v' is its frequency, and 'h' is Planck's constant. | Can be plotted in a wavelength, frequency or wave number. |
Source: https://www.differencebetween.info/difference-between-emission-and-absorption-spectra
0 Response to "Differences Between a Continuous Spectrum an Emission Spectrum and an Absorption Spectrum"
Post a Comment